Preparing samples for microinjection
Why sample quality is important
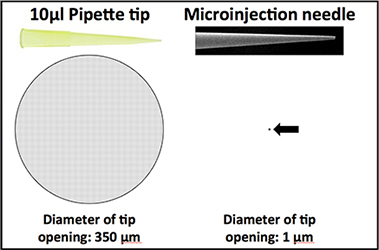
Sample viscosity: When we are ready to use your sample, we transfer some of it to a glass microinjection needle. We then penetrate dechorionated embryos with the needle and use air pressure to force out some of the sample. The microinjection needle tip is several hundred times narrower than a standard pipette tip (see daiagram above). If your sample is viscous or contains any floating particles, it will not pass easily through the needle and your injection may fail. Please note that samples containing plasmids and other small nucleic acid molecules at our recommended concentrations should appear like water and should not be at all viscous.
Sample purity: We inject living embryos with a relatively large amount of fluid. The survival rate of the injected embryos is dependent upon the purity of the sample that is injected. If your sample is unusually toxic, your injection may fail. Please prepare nucleic acids using our recommended procedures below (unfortunately, we are not able to offer this as a service). Ensure that your sample is in pure water and not buffer (e.g TE) as buffers are toxic to the embryo. Also, be aware that high concentrations of nucleic acid (above 1.0 ug/ul) in your sample will compromise embryo survival.
How to prepare plasmid DNA for microinjection
We recommend that plasmid DNA for embryo injection is prepared using the Qiagen mini-, midi- or maxiprep kit. If you use the Qiagen miniprep kit, you need to include the 'optional' PB buffer wash step. The OD260/280 ratio of your DNA sample should be 1.80-1.90. We acknowledge that other kits may generate DNA of the required quality for microinjection, but we will not recommend them until we have established that this is the case.
Update August 2019: Although Qiagen kits can yield DNA that is ideal for microinjection, we have found that some samples made with Qiagen kits have been problematic to inject or appear to have been toxic to the embryos. We do not fully understand why this occurs, but it has become clear that bacterial cultures grown for extended periods, or stored at 4oC before starting the Qiagen prep, can yield DNA samples that are unsuitable for microinjection. This is likely due to harmful secretions by bacteria in stationary phase. We suspect that other variations from the standard Qiagen protocol can also produce problematic samples.
Consequently, our advice is to follow the Qiagen protocol as closely as possible and include any 'optional' washing steps. If possible, complete the prep in a single session avoiding any extended incubation or storage periods.
Adding helper plasmids to your microinjection sample
We can add helper plasmids to your sample to express the folllowing recombinases:
- P-element transposase (pUC hsPI[delta2-3})
- PiggyBac transposase (atub-pBac-K10)
- PhiC31 integrase for attP/attB recombination (act-phiC31-integrase)
Please let us know on our microinjection request form if you want us to add helper plasmid to your sample. Also, please let us know in the 'Comments' section of the request form if you have already added helper plasmid to your sample. It is important for us to have this information as we review all requests for potential problems with stock selection, screening etc.
Recommended sample concentration and volume
Please provide us with at least 20ul of sample in a small (0.5 ml) Eppendorf tube. It is easier for us to work with small volumes in small 0.5ml tubes, which means we are less likely to need to request additional sample if multiple injection series are required. Please do not use PCR tubes as they are fragile and easily crushed
Please send your samples so that they are ready to inject. In this way, we can avoid any errors that may arise when diluting or mixing samples. Our recommended concentrations are:
P-element transgenesis: Please send your P-element plasmid at approximately 1.0 ug/ul. We will added P-element helper plasmid. Final injection concentrations will be 0.8 ug/ul P element plasmid and 0.5 ug/ul helper plasmid.
PiggyBac transgenesis: Please send your PiggyBac plasmid at approximately 0.8 ug/ul. We will added PiggyBac helper plasmid. Final injection concentrations will be 0.6 for PiggyBac plasmid and 0.4 ug/ul helper plasmid.
phiC31 integrase-mediated transgenesis: Around 0.4ug/ul is the most common injection concentration. If you need us to add the integrase helper plasmid, please provide your sample at 0.5 ug/ul. Final injection concentrations will be 0.4 ug/ul for PhiC31 sample plasmid and 0.5 ug/ul PhiC31 integrase plasmid.
CRISPR: For CRISPR mutagenesis, we recomend that your guide plasmid concentrations is 0.1 ug/ul. For CRISPR homologous recombination, we recommend your sample contains 0.1ug/ul guide plasmid and 0.5 ug/ul donor plasmid. Please prepare the donor and guide plasmid mixture before sending your sample.
If you wish to differ from our recommended concentrations, that is fine, but please let us know your alternate concentrations for our records. Also, be aware that over 1.0 ug/ul of sample concentration can compromise embryo survival.
Mailing your sample
If your sample tube is crushed during transport, your sample may be lost through the lid opening or the tube splitting. Therefore, it is best to mail your sample in a padded envelope (Jiffy bag) or a box. Sealing the lid with parafilm or similar will help prevent the lid opening and also guard against evaporation.
